
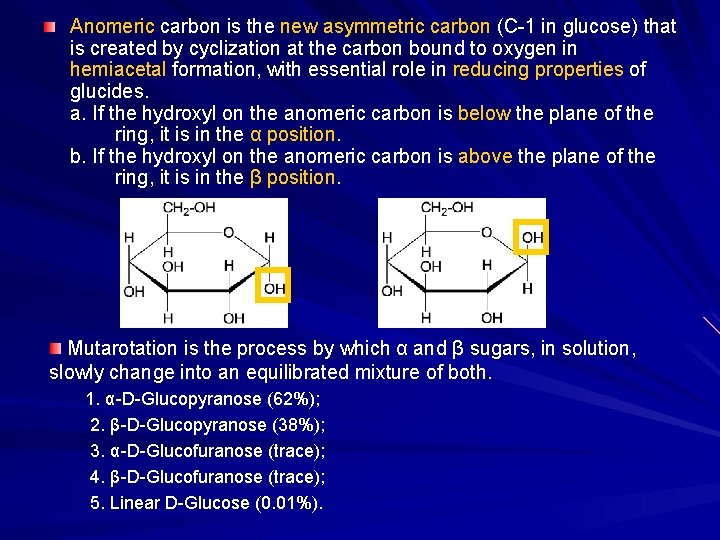
For example, in α- D-glucopyranose the reference atom is C-5. In aldohexoses the anomeric reference atom is the stereocenter that is farthest from anomeric carbon in the ring (the configurational atom, defining the sugar as D or L). The anomeric centre in hemiacetals is the anomeric carbon C-1 in hemiketals, it is the carbon derived from the carbonyl of the ketone (e.g.

Two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the anomeric centre and the anomeric reference atom, hence they are relative stereodescriptors. 1 = Fischer projection with C-1 at the top of the anomeric centre. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations.ĭifferent projections of α-D-glucopyranose. Anomerization is the process of conversion of one anomer to the other. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon in a cyclic saccharide. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon is planar and thus achiral. In carbohydrate chemistry, a pair of anomers (from Greek ἄνω 'up, above', and μέρος 'part') is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. ( Learn how and when to remove this template message)
( May 2011) ( Learn how and when to remove this template message) Please help improve it to make it understandable to non-experts, without removing the technical details. This section may be too technical for most readers to understand.


 0 kommentar(er)
0 kommentar(er)
